详细参数
| 光致发光测量波长范围 | 300-950nm |
|---|---|
| 单色光源 | |
| 光源 | 150W氙灯 |
| 激发波长 | 250-800 nm |
| 带宽 | 10 nm以下(FWHW) |
| 激发波长控制 | 手动 |
| 多通道光谱仪 | |
| 测量波长范围 | 200-950 nm |
| 波长分辨率 | < 2 nm |
| 感光器件通道数 | 1024 ch |
| 制冷温度 | -15 摄氏度 |
| A/D分辨率 | 16 bit |
| 光谱仪类型 | Czerny-Turner型 |
| 光纤类型 | 光纤束(1.5 m) |
| 光纤接收面积 | 直径 1 mm |
| 积分球 | |
| 材料 | Spectralon |
| 尺寸 | 3.3 inch |
| 样品夹持器(可选) | |
| 薄膜 | A10095-01/-03 (不包含基底) |
| 溶液(室温) | 光致发光溶液测量夹持器A10104-01 |
| 溶液(低温) | -196摄氏度(77K)光学低温测量 A11238-01 |
| 温度控制 | 室温(RT)到+180摄氏度带样品夹持器的温度控制 |
| 样品盒(可选) | |
| 粉末 | 采用光致发光粉末测量皿A10095-01/-03 |
| 溶液(室温) | 采用光致发光溶液测量侧臂盒A10095-02 |
| 溶液(低温) | -196摄氏度(77K)采用样品管低温测量A10095-04 |
| 软件 | |
| 测量项目 | 光致发光量子效率 荧光材料发光发光测量(量子效率X吸收) 量子效率和激发波长的关系(-02G,-03G) 光致发光谱(峰值波长,FWHM) 光致发光激发谱(-02G,-03G) 色彩测定(色度、色温、显色指数等) EEM(激发-发射矩阵) |
特性
●测量发光材料光致发光的绝对量子效率
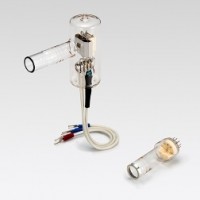
在开发新的发光材料过程中,提高他们的光致发光效率是至关重要的。提高该效率就需要测量量子效率*的精确技术。Quantaurus-QY系统包含了一个氙灯型激发光源、一个单色仪、一个氮气流可选的积分球和一个能同步测量多个波长的多通道探测器,并将所有元件集成到一个封装里。系统采用专用软件用于测量。探测器采用制冷型背照式CCD传感器,能进行高灵敏度的瞬时测量。Quantaurus-QY能处理溶液、薄膜和粉末样品,并能将溶液样品冷却到液氮温度。
*光致发光过程发射光子数与发光材料吸收光子数的比值
●瞬时测量
多通道探测器能捕获灵敏度补偿型光谱,并且通过计算快速获得量子效率数值。对话框型专用软件使得测量过程变得更简单。
●全自动硬件
软件控制的单色仪可以选择激发波长以使样品能被多种波长激发。基于波长的量子效率和激发谱可以自动测定。
●分析不同形式的样品
Quantaurus-QY能处理溶液、薄膜和粉末样品,并能将溶液样品冷却到-196摄氏度(77K)。
●波长范围:300 nm – 950 nm
●测定发光材料的绝对光致发光量子效率(光致发光测量)
●采用积分球测量整个谱域
●制冷型背照式CCD传感器实现超高灵敏度和高信噪比测量
●激发波长的自动控制
●空间集约的紧凑型设计
●可选择多种分析功能
?光致发光的量子效率测量
?激发波长关系
?光致发光谱
?光致发光激发谱
●量子效率测量原理
量子效率和荧光寿命的关系
| 右图的Jablonski能级图描述了普通有机分子的电子能级,并标示了能级间的电子跃迁。S0、S1和T1分别代表基态,最低单态和最低三重态。光激发后,激发态分子可以沿几种跃迁路径,包括辐射过程和非辐射过程而回到基态。辐射过程涉及了光发射,例如荧光和磷光。非辐射过程涉及内转换和系统间热释放。辐射过程和非辐射过程相互竞争。 |
当荧光速率常数、内转换和系统间交换分别用kf, kic, and kisc来简写时,荧光寿命Tf可以用下式表示:
Tf = 1/ (kf + kic + kisc) (1)
同时荧光量子效率Φf可以用下式表示:
Φf = kf / (kf + kic + kisc) (2)
因此等式(3)可以从等式(1)和(2)推导出:
kf = Φf / Tf (3)
从以上的等式可以看出,荧光寿命和量子效率之间有密切的关系。这些参数在控制荧光材料的发光特性上有着基础而重要的作用。
滨松集团开发了Quantaurus系列用于不同的发光材料的评估。现有的Quantaurus-Tau和Quantaurus-QY可分别用于测量荧光寿命和量子效率。这两个系统的支持性分析可以推动用户对光致发光材料的开发。
您可以在下面的推荐产品区域获取紧凑型荧光寿命光谱仪Quantaurus-Tau的细节信息。
应用
量子效率测量能在诸多领域满足开发和研究的应用需求。典型应用包括:包括有机EL材料、白光LED和FPD荧光粉等多种类型的发光材料的性能提升,有机金属复合物的研究,染料敏化型太阳能电池的基础特性评估,生物领域的荧光探针效率测量等。
·有机金属复合物
·荧光探针
·染料敏化型PV材料
·OLED材料
·量子点
·LED荧光粉
测量程序图
分析功能
·激发波长自动扫描
| 左图展示了光致发光量子效率和激发波长的关系。通过机动型单色仪易于测定样本的光致发光量子效率对激发波长的函数关系。 |
|---|
·光致发光的激发谱
| 样品产生的激发谱可以在激发光照射下由机动型单色仪测定。通过选择两条光标线的范围可以轻松获取某个激发波长范围内的光致发光激发谱。 |
|---|
·光致发光谱
| 光致发光谱是在减去激光光后显示的。量子效率测量过程中样品的发光谱线常包含未被样品吸收的激发光成分。减去这种激发光就可以显示仅由样品本身发射的光谱。 |
|---|
·光致发光量子效率测量
| 左图是量子效率测量的基本界面。荧光量子效率在测量后自动计算。激发带和发射带由光标调整来界定。量子效率的数值显示在图表下方,紧邻发光强度、峰值波长、峰值计数和峰值带宽(FWHM)。 |
|---|
·X-Y坐标轴
| 除了显示光致发光谱和计算量子效率,该软件也包括彩色坐标功能。除了被测样品的色度(x,y),三刺激值(X, Y, Z)也被显示。 |
|---|
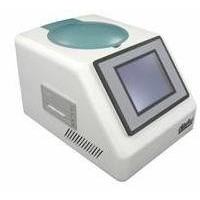
外形尺寸
发表文献
| 应用 | 发表文献 | |||||
| 作者 | 标题 | 期刊名 | 卷号 | 页数 | 年份 | |
| OLEDs | A. Endo, K. Suzuki, T. Yoshihara, S. Tobita, M. Yahiro. and C. Adachi | Measurement of phosphorescence efficiency of Ir(III) phenylpyridine derivatives in solution and solid-state films | Chem. Phy. Lett. | 460 | 155 | 2008 |
| T. Sajoto, P. I. Djurovich, A. B. Tamayo, J. Oxgaard, W. A. Goddard III, and M. E. Thompson | Temperature Dependence of Blue Phosphorescent Cyclometalated Ir(III) Complexes | J. Am. Chem. Soc. | 131 | 9813 | 2009 | |
| H.-F. Chen, S.-J. Yang, Z.-H. Tsai, W.-Y. Hung, T.-C. Wang, and K.-T. Wong | 1,3,5-Triazine Derivatives as New Electron Transport-type Host Materials for Highly Efficient Green Phosphorescent OLEDs | J. Mater. Chem. | 19 | 8112 | 2009 | |
| H. J. Bolink, L. Cappelli, S. Cheylan, E. Coronado, R. D. Costa, N. Lardies, Md. K. Nazeeruddin, and E. Orti | Origin of the Large Spectral Shift in Electroluminescence in a Blue Light Emitting Cationic Iridium(III) Complex | J. Mater. Chem. | 17 | 5032 | 2007 | |
| R. D. Costa, F. J. Cespedes-Guirao, H. J. Bolink, F. Fernandez-Lazaro, A. Sastre-Santos, E. Orti, and J. Gierschner | A Deep-Red-Emitting Perylenediimide-Iridium-Complex Dyad: Following the Photophysical Deactivation Pathways | J. Phys. Chem. C | 113 | 19292 | 2009 | |
| R. D. Costa, F. Fernandez, L. Sanchez, N. Martin, E. Orti, and H. J. Bolink | Dumbbell-Shaped Dinuclear Iridium Complexes and Their Application to Light-Emitting Electrochemical Cells | Chem. Eur. J | 16 | 9855 | 2010 | |
| R. D. Costa, E. Orti, H. J. Bolink, S. Graber, C. E. Housecroft, and E. C. Constable | Efficient and Long-Living Light-Emitting Electrochemical Cells | Adv. Funct. Mater. | 20 | 1511 | 2010 | |
| R. D. Costa, E. Orti, D. Tordera, H. J. Bolink, S. Graber, C. E. Housecroft, L. Sachno, M. Neuburger, and E. C. Constable | Stable and Efficient Solid-State Light-Emitting Electrochemical Cells based on a Series of Hydrophobic Iridium Complexes | Adv. Funct. Mater. | 1 | 282 | 2011 | |
| 荧光粉 | T. Nakajima, M. Isobe, T. Tsuchiya, Y. Ueda, and T. Kumagai | Direct fabrication of metavanadate phosphor films on organic substrates for white-light-emitting devices | Nature Materials | 7 | 735 | 2008 |
| T. Ogi, Y. Kaihatsu, F. Iskandar, W.-N. Wang, and K. Okuyama | Facile Sunthesis of New Full-Color-Emitting BCNO Phosphors with High Quantum Efficiency | Adv. Mater | 20 | 3235 | 2008 | |
| 荧光探针 | H. Ito, M. Matsuoka, Y. Ueda, M. Takuma, Y. Kudo, and K. Iguchi | Quinolinecarboxylic acid based fluorescent molecules: ratiometric response to Zn2+ | Tetrahedron | 65 | 4235 | 2009 |
| S. Kamino, H. Ichihara, S. Wada, Y. Horio, Y. Usami, T. Yamaguchi, T. Koda, A. Harada, K. Shimanuki, M. Arimoto, M. Doi, and Y. Fujita | Degign and Synthesis of Regioisomerically Pure unsymmetrical Xanthene Derivatives for Staining live Cells and Their Photochemical Properties, | Bioorg. Med. Chem. Lett. | 18 | 4380 | 2008 | |
| Y. Mikata, A. Yamashita, A. Kawamura, H. Konno, Y. Miyamoto, and S. Tamotsu | Bisquinoline-based fluorescent zinc sensors | Dalton Trans. | 3800 | 2009 | ||
| Takahisa Suzuki, Seisuke Arai, Mayumi Takeuchi, Chiye Sakurai, Hideaki Ebana, Tsunehito Higashi, Hitoshi Hashimoto, Kiyotaka Hatsuzawa, Ikuo Wada | Development of Cysteine-Free Fluorescent Proteins for the Oxidative Environment | PLoS ONE | 7 | e37551 | 2012 | |
| 有机复合物 | K. Suzuki, A. Kobayashi, S. Kaneko, K. Takehira, T. Yoshihara, H. Ishida, Y. Shiina, S. Oishi, and S. Tobita | Reevaluation of Absolute Luminescence Quantum Yields of Standard Solutions Using a Spectrometer with an Integrating Sphere and a Back-Thinned CCD Detector | Phys. Chem. Chem. Phys. | 11 | 9850 | 2009 |
| R. Kato, K. Suzuki, A. Furube, M. Kotani, and K. Tokumaru | Fluorescence quantum yield of aromatic hydrocarbon crystals | J. Phys. Chem. C | 113(7) | 2961 | 2009 | |
| N. Hayashi, Y. Saito, H. Higuchi, and K. Suzuki | Comparative Studies on Electronic Spectra and Redox Behaviors of Isometric Benzo[1,2-b:4,5-b’] difurans and Benzo[1,2-b:5,4-b’]difrans | J. Phys. Chem. A | 113(18) | 5342 | 2009 | |
| K. Tani, C. Ito, Y. Hanaka, M. Uchida, K. Otaguro, H. Horiuchi, and H. Hiratsuka | Photophysical Property and Photostability of J-Aggregate Thin Films of Thiacyanine Dyes Prepared by the Spin-Coating Method, | J. Phys. Chem. B | 112(3) | 836 | 2008 | |
| M. Shimizu, K. Mochida, and T. Hiyama | Modular Approach to Silicon-Bridged Biaryls: Palladium-Catalyzed Intramolecular Coupling of 2-(Arylsilyl)aryl Triflates | Angew. Chem. Int. Ed | 47 | 9760 | 2008 | |
| M. Shimizu, Y. Takeda, M. Higashi, and T. Hiyama | 1,4-Bis(alkenyl)-2,5- dipiperidinobenzenes: Minomal Fluorophores Exhibiting Highly Efficient Emission in the Solid State | Angew. Chem. Int. Ed | 48 | 3635 | 2009 | |
| A. Fukazawa, M. Hara, T. Okamoto, E.-C. Son, C. Xu, K. Tamao, and S. Yamaguchi | Bis-Phosphoryl-Brigged Stilbenes Synthesized by an Intramolecular Cascade Cyclization, | Org. Lett | 10(5) | 913 | 2008 | |
| C.-H. Zhao, A. Wakamiya, Y. Inukai, and S. Yamaguchi | Highly Emissive Organic Solids Containing 2,5-Diboryl-1,4-phenylene Unit | J. Am. Chem. Soc. | 128 | 15934 | 2008 | |
| 金属-有机化合物 | A. Ishii, K. Habu, S. Kishi, H. Otsu, T. Komatsu, K. Osaka, K. Kato, S. Kimura, M. Tanaka, M. Hasegawa, and Y. Shigesato | Novel Emission Properties of Melem Caused by the Heavy metal Effect of Lanthanides(III) in a LB Film | Photochem. Photobiol. Sci. | 6 | 804 | 2007 |
| K. Matsumoto, N. Matsumoto, A. Ishii, T. Tsukuda, M. Hasegawa, and T. Tsubomura | Structual and Spectroscopic Properties of a Copper(I)-bis(N-heterocyclic)carbene Complex | Dalton Trans. | 6795 | 2009 | ||
| Y. Matano, T. Miyajima, N. Ochi, Y. Nakao, S. Sakai, and H. Imahori | Synthesis of Thiophene-Containing Hybrid Calixphyrins of the 5,10-Porphodimethene Type | J. Org. Chem. | 73(13) | 5139 | 2008 | |
| D. Kuzuhara, J. Mack, H. Yamada, T. Okujima, N. Ono, and N. Kobayashi | Synthesis, Structures, and Optical and Electrochemical Properties of Benzoporphycenes | Chem. Eur. J | 15 | 10060 | 2009 | |
| D. Maeda, H. Shimakoshi, M. Abe, M. Fujitsuka, T. Majima, and Y. Hisaeda | Synthesis of a Novel Sn)IV) Porphycene-Ferrocene Triad linked by Axal Coordination and Solvent Polarity Effect in Photoinduced Charge Separation Process | Inorg. Chem. | 49 | 2872 | 2010 | |
| D. Maeda, H. Shimakoshi, M. Abe, and Y. Hisaeda | Synthesis and photophysical behavior of porphyrin isomer Sn(IV) complexes | Inorg. Chem. | 48 | 9853 | 2009 | |
| H. Shimakoshi, T. Baba, Y. iseki, I. Aritome, A. Endo, C. Adachi, and Y. Hisaeda | Photophysical and photosensitizing properties of brominated porphycenes | Chem. Commun. | 2882 | 2008 | ||
注:该仪器未取得中华人民共和国医疗器械注册证,不可用于临床诊断或治疗等相关用途









 拨打电话
拨打电话 发送询价
发送询价